KEYTRUDA® in combination with LENVIMA® is indicated for:
- the treatment of adult patients with advanced (not amenable to curative surgery or radiation) or metastatic RCC with no prior systemic therapy for metastatic RCC.
Kidney Cancer Research Network of Canada (KCRNC) 2021 consensus statement3
In untreated patients with advanced clear cell renal cell carcinoma with favourable, intermediate, or poor risk (IMDC), pembrolizumab + lenvatimib is recommended as a preferred treatment option
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines for kidney cancer (NCCN Guidelines®)5
In patients with Stage IV clear-cell RCC, pembrolizumab + lenvatinib is recommended as a first-line preferred option across all favourable-, intermediate-, and poor-risk groups.
Clinical Study
KEYNOTE-581/CLEAR trial: clinical study in patients with advanced or metastatic RCC.
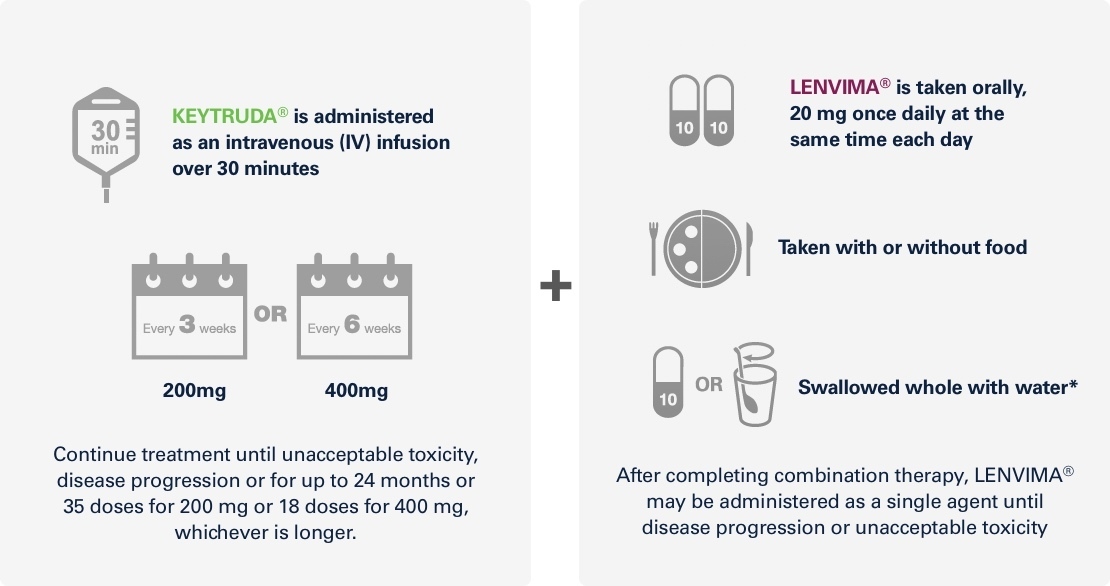
KEYNOTE-581/CLEAR trialKEYTRUDA® + LENVIMA® recommended dosing:
For adult patients with advanced or metastatic RCC with no prior systemic therapy for metastatic RCC1,2
Optimal medical management (i.e. treatment or therapy) for nausea, vomiting, and diarrhea should be initiated prior to any LENVIMA® therapy interruption or dose reduction; gastrointestinal toxicity should be actively treated in order to reduce the risk of development of renal impairment or failure.2
Electrolytes, liver enzymes, urinary protein, thyroid function and hypertension should be tested prior to LENVIMA® treatment and monitored periodically during LENVIMA® therapy.2
Caregivers should not open the LENVIMA® capsule, in order to avoid repeated exposure to the contents of the capsule.2
Dosing modifications
When administering LENVIMA® in combination with KEYTRUDA®, interrupt one or both drugs, dose reduce or discontinue LENVIMA® as appropriate. Withhold or discontinue KEYTRUDA® in accordance with the instructions in the KEYTRUDA® Product Monograph. No dose reductions are recommended for KEYTRUDA®.1,2
Please see the Product Monograph for complete dosing and administration recommendations.
KEYTRUDA®+ LENVIMA® adverse event (AE) management tools
For guidance on AE management in patients taking KEYTRUDA®+ LENVIMA®, download this handbook.
Help your patients taking KEYTRUDA®+ LENVIMA® keep track of their side effects by providing this personal diary.
cHL=classical Hodgkin lymphoma; PMBCL=Primary mediastinal B cell lymphoma; AE=adverse event; IMDC=International Metastatic RCC Database Consortium.
* Alternatively, the LENVIMA® capsules may be added without breaking or crushing them to a tablespoon of water or apple juice in a small glass to produce a suspension. The capsules must be left in the liquid for at least 10 minutes and stirred for at least 3 minutes to dissolve the capsule shells. The suspension is to be swallowed. After drinking, the same amount of water or apple juice (one tablespoon) must be added to the glass and swirled a few times. The additional liquid must be swallowed.2
References:
- Merck Canada Inc. KEYTRUDA® Product Monograph. March 21, 2024.
- Eisai Limited. LENVIMA® Product Monograph. July 19, 2023.
- Canil C et al. Management of advanced kidney cancer: Kidney Cancer Research Network of Canada (KCRNC) consensus update 2021. Can Urol Assoc J. 2021;15(4):84‒97.
- Medical Council of Canada. Clinical laboratory tests: Adult normal values. Available at: https://mcc.ca/examinations-assessments/resources-to-help-with-exam-prep/normal-lab-values/. Last accessed April 19, 2023.
- NCCN Clinical Practice Guidelines in Oncology. Kidney Cancer, Version 2.2024.
- Motzer R et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi: 10.1056/NEJMoa2035716
CA-KLH-00054
